
Antigen-specific stimulation
To assess antigen-specific responses, lymphocytes are activated in an antigen-specific manner via their T or B cell receptor during in vitro cultures. Common antigens to which most humans have developed T cell memory can be used for these assays, such as antigens present in vaccines or pathogens, such as CMV, EBV, Flu, Tetanus toxoid, or a combination thereof.
Questions that can be answered using this type of assay include whether memory responses to therapeutic/vaccine proteins occur or can be induced in human healthy volunteers or patients. Furthermore, whether an immunomodulatory compound affects antigen-specific responses can be determined .
Antigen-specific responses can either be investigated in total PBMC cultures or in co-cultures of monocyte-derived dendritic cells with isolated autologous T cells. If required, these cultures can be performed with healthy donors of a specific HLA type, which may be particularly interesting when testing a vaccine. We can either repeatedly use the same donor or use several different donors. Furthermore, we have a selection of donors available who are responsive to specific antigens.
 Depending on your research question, we offer several different types or read-outs indicative for antigen-specific lymphocyte activation. These include antibody production analysis, cellular phenotyping, cytokine production analysis and proliferation assays.
Depending on your research question, we offer several different types or read-outs indicative for antigen-specific lymphocyte activation. These include antibody production analysis, cellular phenotyping, cytokine production analysis and proliferation assays.
Examples of antigen-specific T cell activation
Assessing the amount of antigen-specific T cells in a PBMC sample via ELIspot
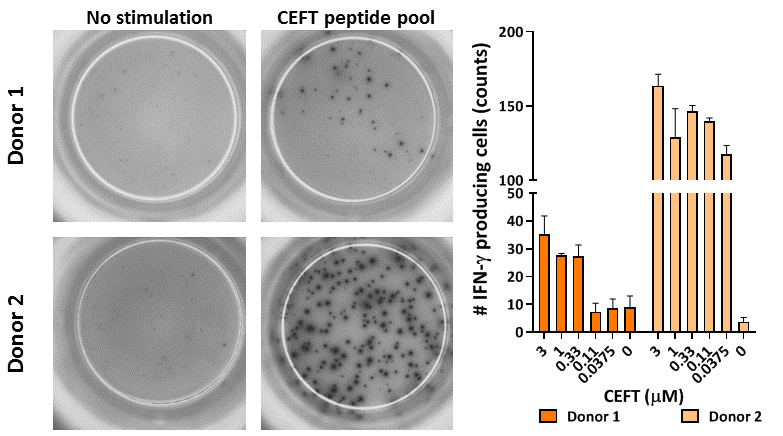 PBMC were thawed and left to rest overnight. Subsequently, the cells were stimulated for 16 hours with a CEFT peptide pool containing CMV, EBV, Flu and Tetanus toxoid peptides. Afterwards, IFN-γ production was assessed via ELIspot. Left, representative images for unstimulated and CEFT-stimulated wells, right, compilation data of IFN-γ producing cells from 2 donors upon increasing concentrations of CEFT (3 replicates per condition, mean count ± standard deviation).
PBMC were thawed and left to rest overnight. Subsequently, the cells were stimulated for 16 hours with a CEFT peptide pool containing CMV, EBV, Flu and Tetanus toxoid peptides. Afterwards, IFN-γ production was assessed via ELIspot. Left, representative images for unstimulated and CEFT-stimulated wells, right, compilation data of IFN-γ producing cells from 2 donors upon increasing concentrations of CEFT (3 replicates per condition, mean count ± standard deviation).
Assessing T cell proliferation after dendritic cell immunogenic peptide presentation via 3H thymidine incorporation
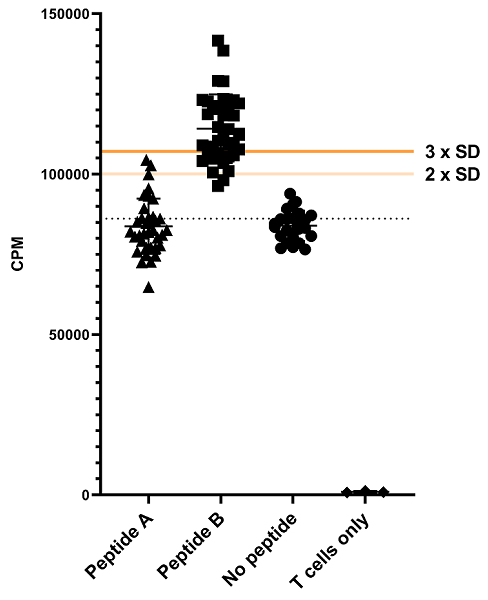
The T cell response to immunogenic peptides presented by dendritic cells (DC) was assessed using 3H thymidine incorporation as a read-out. After loading the DCs with the peptides, T cells were added to the culture. Proliferation during 24 hours was measured after 7 days of co-culture. A no peptide control was included to correct for basal T cell proliferation in this set up. A response to the presented peptide was defined as values >3 SD above the no peptide control.
For more applications, please see the references below.
References
Guidelines for analysis of low-frequency antigen-specific T cell results: Dye-based proliferation assay vs 3 H-thymidine incorporation
Di Blasi D, Claessen I, Turksma AW, van Beek J, ten Brinke A.
J Immunol Methods 2020;487:112907. doi: 10.1016/j.jim.2020.112907.
Monitoring T-Cell Responses in Translational Studies: Optimization of Dye-Based Proliferation Assay for Evaluation of Antigen-Specific Responses
ten Brinke A, Marek-Trzonkowska N, Mansilla MJ, Turksma AW, Piekarska K, Iwaszkiewicz-Grześ D, Passerini L, Locafaro G, Puñet-Ortiz J, van Ham SM, Hernandez-Fuentes MP, Martínez-Cáceres EM, Gregori S.
Front Immunol 2017;8:1870. doi: 10.3389/fimmu.2017.01870.
Employ antigen-specific activation
We offer antigen-specific activation for all lymphocyte subsets.
Read-out options
We offer several different read-out options for antigen-specific lymphocyte activation, but feel free to contact us for an assay customized to your needs.
Other stimulation assays
Besides antigen-specific stimulation, we also offer other stimulation assays. In addition to antibody-mediated receptor crosslinking and chemical stimulation, please find a selection of our assays below.

