Regulations
Regulatory views on MATA brief history on pyrogen testing
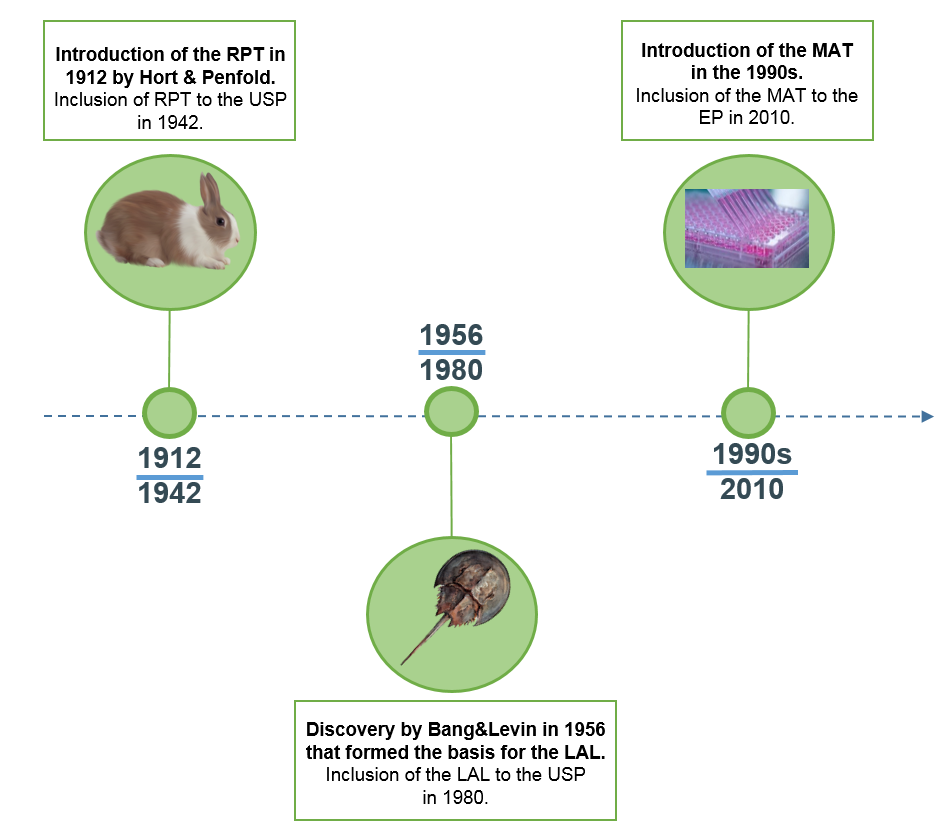
In 1912, Hort and Penfold were the first to develop a pyrogen test which was based on parenteral administration of pharmaceuticals into rabbits and measuring the possible rise in body temperature, known as the Rabbit Pyrogen Test (RPT). In 1942, the test was included in the 12th edition of the United States Pharmacopeia (USP) as a pyrogen test.
Click on the image to enlarge.
In 1956, research by Bang and Levin formed the basis for the development of an alternative test to the RPT. The investigators found that the blood of the horseshoe crab forms a clot when exposed to Gram-negative bacteria. Based on this discovery, the Limulus Amebocyte Lysate (LAL) assay was developed using extracts of amebocytes from limulus blood to analyze pharmaceuticals for endotoxin contamination. the LAL test was incorporated in the 20th edition of the USP in 1980. Although the LAL test proved to be very sensitive to endotoxin and easy to use, its inability to detect non-endotoxin pyrogens (NEPs) prevented the assay to fully replace the RPT.
The limitations of the RPT (low sensitivity, not quantitative) and LAL (inability to detect NEP), and the increasing concerns on animal welfare, pushed the development of an alternative pyrogen test. Based on the human immune response towards pyrogens, an in vitro-based assay was developed known as the Monocyte Activation Test (MAT) and incorporated in the European Pharmacopeia (EP) in 2010. Sanquin was the first to release a commercially available, highly sensitive MAT kit based on cryopreserved PBMCs in 2017 and since then offers both MAT assay kits and test services.
Guidelines for pyrogen detection in pharmaceutical and biological products
Europe:
- EP 2.6.8 pyrogens: recommendations to replace Rabbit Pyrogen Test by MAT (2.6.30) wherever possible (July 2016);
- EP 5.1.10 Guidelines for using the test for bacterial endotoxins specifies: "The Monocyte activation test (2.6.30) is a suitable method to use to rule out the presence of non-endotoxin pyrogens in substances or products" (January 2017);
- EP 2.6.30 Monocyte Activation Test (January 2017): in the guidance notes, it is mentioned: "The monocyte activation test (MAT) is primarily intended to be used as an alternative method to the rabbit pyrogen test." This chapter has recently been revised to include the need to use Non-Endotoxin Pyrogens (NEPs) as positive control.
USA:
- FDA "Guidance For Industry Pyrogen and Endotoxins testing: Questions and Answers" 2012: the possible use of Monocyte Activation Test is mentioned as an alternative to the rabbit test but should be validated according to USP <1225>;
- USP <151> Pyrogen Test mentions that "A validated, equivalent in vitro pyrogen or bacterial endotoxin test may be used in place of the in vivo rabbit pyrogen test, where appropriate".
Other countries including India, Japan and China are working on MAT monographs.
Guidelines for pyrogen detection in medical devices
- Revision of ISO/DTR 21852 Pyrogenicity "Principle and method for pyrogen testing of medical devices". The MAT is mentioned as a pyrogen test.
- ISO 10993-1 "Biological evaluation of medical devices – part 11: Test for systemic pyrogenicity." Only the Rabbit Pyrogen Test is recommended because alternative tests were not validated – Published in 2006