Bioanalysis of biologics
Contract research
We offer support during drug development by facilitating various steps in your clinical trial program.
Finger prick
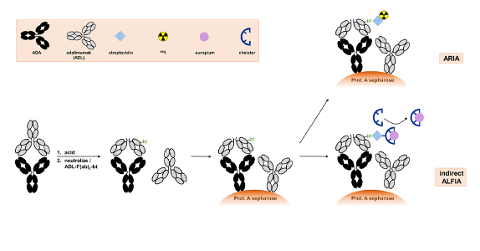
To support Therapeutic Drug Monitoring for biologics, Sanquin Diagnostic Services now offers the possibility to determine the biologic level remotely.
Why drug monitoring
Optimize efficacy of treatment by monitoring drug levels and antibody response elicited by biologics.
Use our expertise
Knowledge institute
Find out what we can do for you as a knowledge and expertise centre in the field of biologics.
Learn more