Scientific Integrity Program
The CCTR Scientific Integrity Program’s primary goal was quality, integrity and reproducibility of all departmental scientific output.
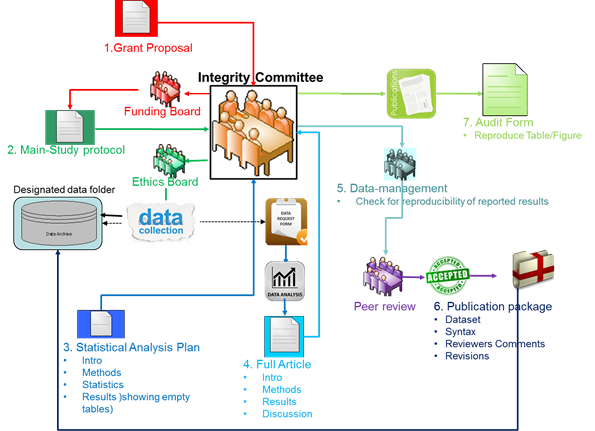
The program was aimed at the regulation and review of the critical phases of clinical research including; grant proposals before review by potential funders, main study protocols before submission to ethics committee, and for each individual type of article (Original, Methodological, Systematic review) both “Statistical Analysis Plan” (SAP) and the “full article” before submission for peer review. Data integrity is regulated through the processing of data requests only after SC approval of the SAP and by detailed documentation of all data manipulations and statistical testing in a syntax. Reproducibility was regulated by checking the syntax for reproducibility of reported results before submission for peer review and archive of the publication package after the publication has been accepted for publication. The program was supported by the Scientific committee. A graphic presentation of the program is shown below.
Past Scientific Committee members (alphabetical order)
- M Baysan MD PhD Student
- C Caram-Deelder PhD
- L Cornelissen MD PhD
- Prof M de Haas MD PhD
- A Gillissen MD
- Á Honohan MSc
- F Kranenburg MD
- S Kroes PhD student
- R Middelburg PhD
- J Oud PhD student
- N Saadah MD PhD
- S Valk PhD student
- L van de Watering MD PhD
- Prof JG van der Bom MD PhD
- M van Kraaij MD PhD
- Prof JJ Zwaginga MD PhD