SUPPLY Project kick-off: EBA leads EU project to strengthen voluntary non-remunerated plasma collection capacity in Europe
NewsThe SUPPLY Project on increasing plasma collection in Europe, representing the first united effort across the EU to collect, investigate, assess, and put forward best practices on how to successfully increase plasma collection in the EU, by not-for-profit blood establishments, has started.
The SUPPLY Project consortium (figure 1 below) kicks off the project today (26 September) in Sanquin HQ in Amsterdam and is comprised of broad and complementary expertise relating to all aspects in blood and plasma collection, processing, and medicinal use. The project represents a significant investment of €1.9Million towards making the EU more strategically independent in its need for plasma medicines, with the European Commission providing a grant of €1.1Million through the EU4Health programme and €756.000 coming from the project consortium members, including not-for-profit blood establishments (BEs).
PDMPs
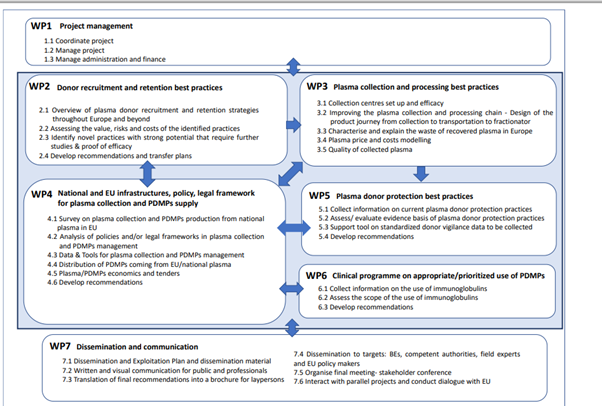
Plasma-derived medicinal products (PDMPs) are used in immunology, haemostasis and intensive care to treat rare, serious, genetic and – in many cases – life-threatening diseases or conditions. These include over 80 recognised Primary Immunodeficiency Diseases, bleeding disorders such as haemophilia and von Willebrand disease, and other genetic disorders relating to missing or non-functioning proteins typically found in blood plasma. The SUPPLY Project, led by the European Blood Alliance (EBA), will ultimately strengthen the resilience of plasma collection in the EU to enable a stable and adequate supply of these medicines in Europe (figure 2 below).
The Covid-19 crisis has shown the vulnerability of the global supply of life-saving medicines, notably PDMPs. Simultaneously, there is an imbalance in the global collection of plasma needed to produce PDMPs, with a high dependence of plasma coming from the U.S. The SUPPLY Project will ensure good practices to both optimise the supply of plasma donated by fellow citizens and increase access to the quality and safe use of PDMPs are shared with medical organisations, other plasma collectors, and EU Member States authorities in substances of human origin subsectors.
Competent Authorities
The SUPPLY Project consortium is also meeting with national competent authorities this week. The SUPPLY Project will provide direct guidance to competent authorities for defining, improving, and implementing specific policies relating to plasma collection and PDMPs management.
For national competent authorities, the SUPPLY Project will provide an assessment of measures on donor deferrals and donor selection criteria, which is particularly important in times of crisis, when potential donors may encounter barriers to donating (e.g., lockdowns, restrictions to movement) and are therefore less numerous than in normal times.
Recommendations on the optimal legal framework will enable competent authorities to make plasma collection programmes possible. Additionally, the SUPPLY Project will also deliver recommendations on the potential impact of turning existing whole blood donors into plasma donors that will help blood establishments and competent authorities define a common strategy on recruitment and retention that will not negatively impact whole blood collection.
“It is essential that the European Union increases its collection of plasma and production of PDMPs to meet the needs of patients and the not-for-profit blood establishments are instrumental in reaching this goal” said Daphne Thijssen-Timmer, Sanquin interim CEO, Vice-President of EBA, and Leader of the SUPPLY Project. “We have positive experience led by the not-for-profit sector (respecting the principle of voluntary unpaid donations) of implementing programmes and strategies that have proven efficient in increasing the volume of plasma collection; it is important that we pool knowledge and expertise built by blood establishments to guide future developments at EU level”, Thijssen-Timmer added.
The project will evaluate current legal frameworks and policies in the EU on plasma collection and tender models to facilitate this process. Since plasma demand comes from the use of immunoglobulins, it is vital that its use is appropriate and prioritisation is properly guided.
The main project outcome is a set of recommendations and guidance for not-for-profit blood establishments, competent authorities, medical societies and other professional stakeholders to support them in being able to increase plasma collection in the EU by the public health sector and achieve optimal availability of PDMPs for patients both in a general situation as well as in times of crises. This project will therefore contribute to the EU becoming more strategically independent in its need for PDMPs.
Figure 1: SUPPLY Consortium
|
Participant organisation name |
Country |
|
European Blood Alliance |
The Netherlands |
|
Etablissement Français du Sang |
France |
|
Stichting Sanquin Bloedvoorziening |
The Netherlands |
|
Belgische Rode Kruis |
Belgium |
|
Universitaet Hamburg |
Germany |
|
DRK-Blutspendedienst Baden-Wurttemberg-Hessen GGMBH |
Germany |
|
European Hematology Association |
The Netherlands |
|
International Plasma and Fractionation Association |
The Netherlands |
|
Bloddonorerne I Danmark |
Denmark |
|
Istituto superiore di sanita |
Italy |
|
The Common Services Agency acting through its strategic business unit The Scottish National Blood Transfusion Service |
UK |
|
International Federation of Blood Donor Organization (FIODS) |
Italy/ Monaco |
|
Servicio Vasco de Salud Osakidetza |
Spain |
|
Irish Blood Transfusion Service |
Ireland |
|
Aarhus Universitet Hospital |
Denmark |
|
Osterreichisches Rotes Kreuz |
Austria |
|
Zavod Republike Slovenije Za Transfuzijsko Medicino |
Slovenia |
|
Ministerio De Sanidad |
Spain |
|
PHI Institute for Transfusion Medicine of RNM |
North Macedonia |
|
Instituto Portugues do Sangue e da Transplantacao IP |
Portugal |
Figure 2: SUPPLY Work Packages (WP)
 The content of this communication represents the views of the author only and his/her sole responsibility; it cannot be considered to reflect the views of the European Health and Digital Executive Agency or any other body of the European Union. The European Commission and the Agency do not accept any responsibility for use that may be made of the information it contains. This communication is part of the project ‘101056988’ which has received co-funding from the EU4Health Programme of the European Union.
The content of this communication represents the views of the author only and his/her sole responsibility; it cannot be considered to reflect the views of the European Health and Digital Executive Agency or any other body of the European Union. The European Commission and the Agency do not accept any responsibility for use that may be made of the information it contains. This communication is part of the project ‘101056988’ which has received co-funding from the EU4Health Programme of the European Union.

